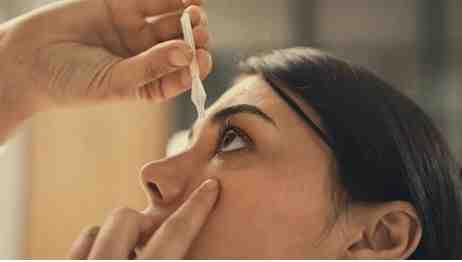
DCGI nod for eye drop to do away with glasses
ENTOD Pharmaceuticals has received final approval from the Drugs Controller General of India (DCGI) for PresVu eye drops, marking a significant milestone in the management of presbyopia.
This approval follows an earlier recommendation by the Subject Expert Committee of the Central Drugs Standard Control Organisation, a company statement said.
PresVu is the first eye drop in India developed to reduce dependency on reading glasses for those affected by presbyopia, a common age-related vision condition, the company said.
Presvu has also applied for a patent for the invention in terms of its formulation and the process. These eye drops utilise advanced dynamic buffer technology to swiftly adapt to tear pH, ensuring consistent efficacy and safety for extended use.
Source : The Times of India