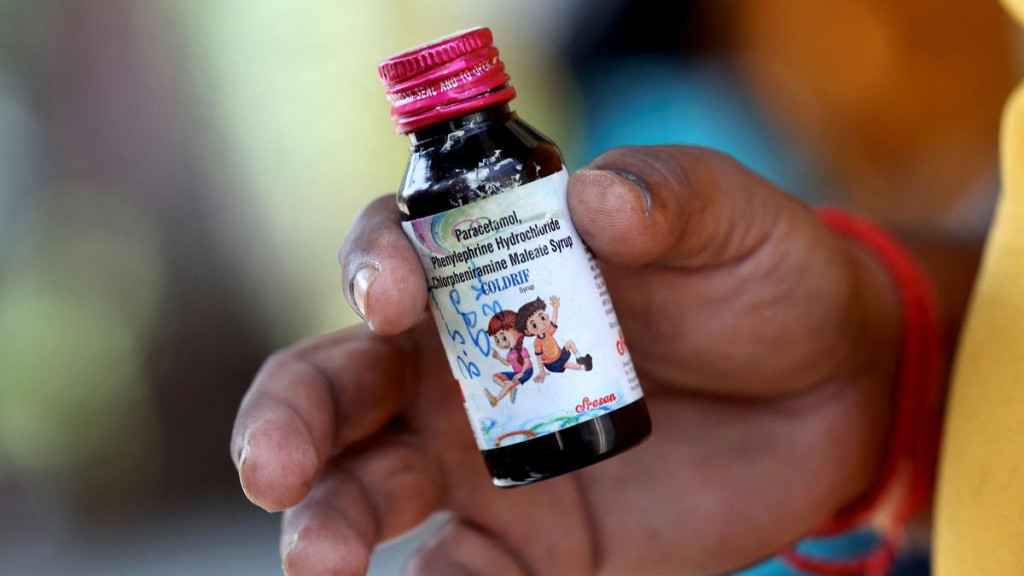
WHO issues medical product alert on three contaminated oral liquid medicines in India
World Health Organisation (WHO) has issued medical product alert on three contaminated oral liquid medicines identified in India and reported to WHO on October 8, 2025. The contaminated oral liquid medicines have been identified to be specific batches of COLDRIF, Respifresh TR and ReLife, manufactured by Sresan Pharmaceutical, Rednex Pharmaceuticals, and Shape Pharma.
The U.N. agency said that the Indian National Regulatory Authorities (NRAs) has been advised to consider targeted market surveillance, with particular attention to informal and unregulated supply chains where products may circulate undetected. NRAs are also advised to carefully evaluate the risks associated with any oral liquid medicines originating from the same manufacturing sites — particularly those produced since December 2024.
source:thehindu.com